Your Location:Home > Products > organic chemicals > 2-(2-Methoxyethoxy)ethanol(DM)
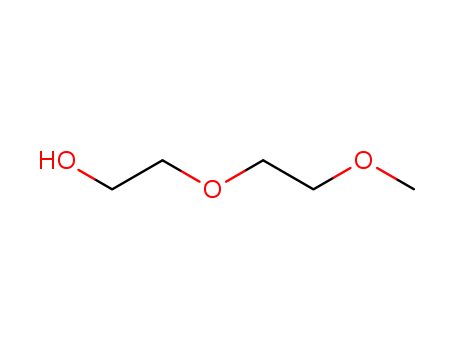


CasNo: 111-77-3
MF: C5H12O3
Appearance: colorless liquid
|
Preparation |
A by-product in the manufacture of ethylene glycol monomethyl ether (Arctander, 1969). |
|
Air & Water Reactions |
Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979 p.151-154, 164]. Water soluble. |
|
Reactivity Profile |
2-(2-Methoxyethoxy)ethanol is a ether-alcohol derivative. The ether being relatively unreactive. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert alcohols to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. |
|
Health Hazard |
DGME is a mild to moderate toxicant via ingestion or absorption through the skin. High doses produced lowering of hemoglobin levels and increased relative kidney weight. Renaldamagemay occurnear the lethal dose. Eye contact of the liquid can result in mild to moderate irritationLD50 value, oral (guinea pigs): 4160 mg/kgPreliminary developmental toxicity test in pregnant mice dosed with DGME indicated teratogenicity of this compound (Hardin et al. 1987; Cheever et al. 1988). Earlier, Doe (1984) reported no teratogenic property of DGME when administered subcutaneously in rats up to 100 mL/kg. In comparison, EGME produced effects at 40 mL/kg.. |
|
Fire Hazard |
2-(2-Methoxyethoxy)ethanol is combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Moderately toxic by skin contact and intraperitoneal routes. Mildly toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. An eye irritant. Combustible when exposed to heat or flame; can react with oxidzing materials. Reacts violently with Ca(OCl)2, chlorosulfonic acid, and oleum. To fight fire, use dry chemical, alcohol foam, water spray or mist, CO2. When heated to decomposition it emits acrid smoke and irritating fumes. See also GLYCOL ETHERS. |
|
Purification Methods |
Purify as for diethylene glycol mono-n-butyl ether. [Beilstein 1 IV 2392.] |
|
Waste Disposal |
DGME is mixed with a combustible solvent and burned in a chemical incinerator. |
|
Definition |
ChEBI: A hydroxypolyether that is the monomethyl ether derivative of diethylene glycol. |
|
General Description |
Colorless liquid with a sweet odor. Floats and mixes with water. |
InChI:InChI=1/C5H12O3/c1-5(7)4-8-3-2-6/h5-7H,2-4H2,1H3
-
Disclosed is a process for recovering fo...
The present invention provides a compoun...
A preparatively facile, highly selective...
The radiolysis of diethylene glycol dime...

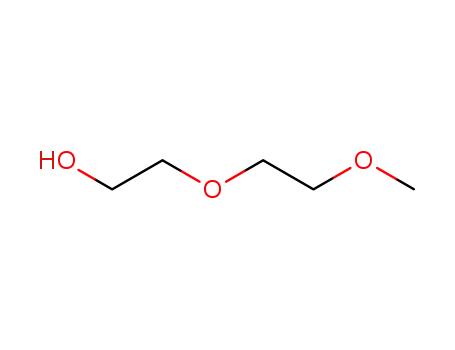
2-(2-methoxyethoxy)ethyl alcohol

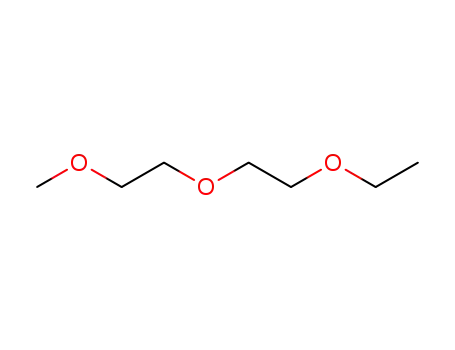
diethylene glycol ethyl methyl ether
| Conditions | Yield |
|---|---|
|
With
nickel;
at 170 - 210 ℃;
Hydrogenation.unter Druck;
|
![1,1-bis-[2-(2-methoxy-ethoxy)-ethoxy]-ethane](/upload/2025/4/917028a0-5dc4-43c6-95e9-c79222af238e.png)
1,1-bis-[2-(2-methoxy-ethoxy)-ethoxy]-ethane


2-(2-methoxyethoxy)ethyl alcohol


diethylene glycol ethyl methyl ether
| Conditions | Yield |
|---|---|
|
at 170 - 210 ℃;
under 51485.6 Torr;
Hydrogenation;
|
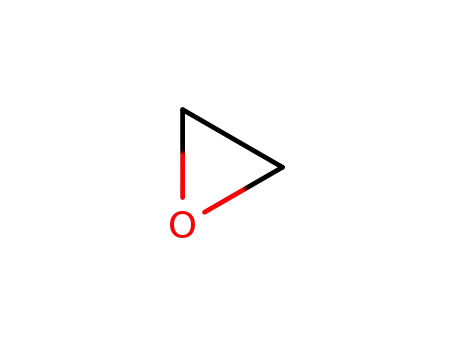
oxirane
methanol
α-naphthol
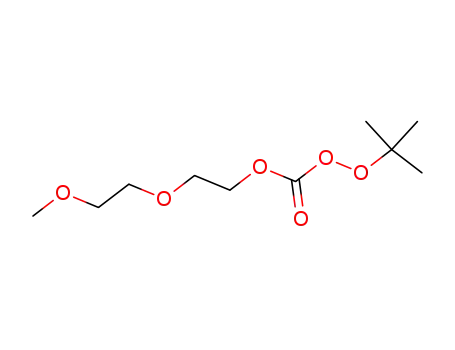
OO-tert-butyl methoxyethoxyethyl monoperoxycarbonate
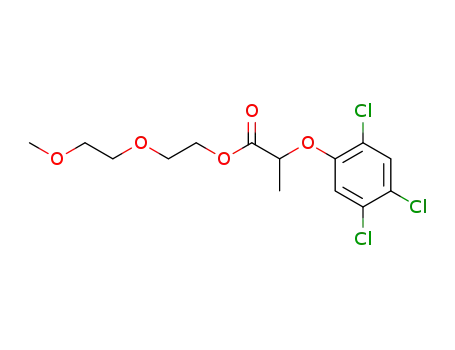
2-(2,4,5-trichloro-phenoxy)-propionic acid-[2-(2-methoxy-ethoxy)-ethyl ester]
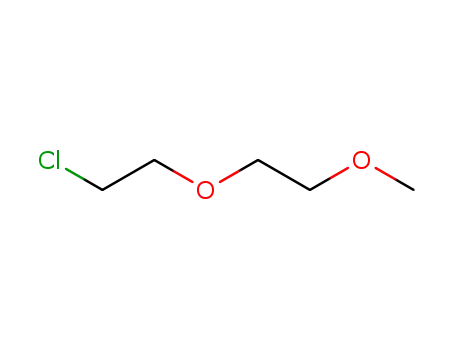
1-chloro-2-(2-methoxyethoxy)ethane
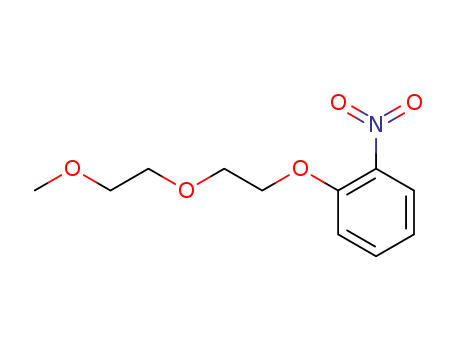
1-(2-(2-methoxyethoxy)ethoxy)-2-nitrobenzene
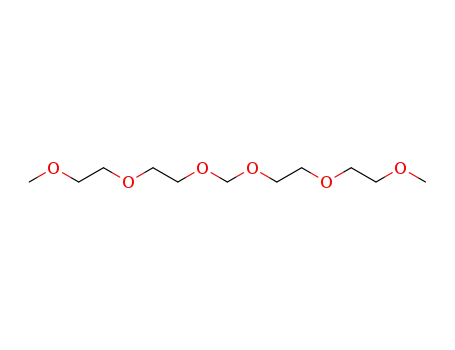
1,13-dimethoxy-3,6,8,11-tetraoxa-tridecane