Your Location:Home > Products > organic chemicals > 1,2-Dimethoxyethane (EDM)
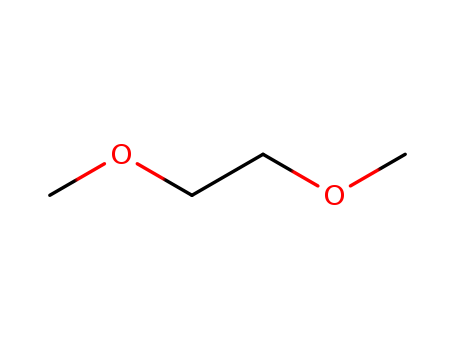


CasNo: 110-71-4
MF: C4H10O2
Appearance: water-white Liquid
|
Synthesis |
1,2-Dimethoxyethane is derived from the reaction of ethylene glycol monomethyl ether with sodium metal and methyl chloride. The ethylene glycol monomethyl ether and the metal sodium were refluxed together until the metal sodium was completely reacted, the temperature was lowered to 45° C., and methyl chloride was introduced. After the reaction is completed, fractional distillation is performed to collect fractions at 84-85.5°C to obtain 1,2-Dimethoxyethane. |
|
Definition |
ChEBI: A diether that is the 1,2-dimethyl ether of ethane-1,2-diol. |
|
General Description |
A liquid with a sharp odor. Less dense than water. Flash point 34°F. Mildly toxic by ingestion and inhalation. Severely irritates skin and eyes. Vapors heavier than air. Used to make other chemicals. |
|
Air & Water Reactions |
Highly flammable. Slightly soluble in water. |
|
Reactivity Profile |
When the solvent, 1,2-Dimethoxyethane, was poured into a funnel previously used to introduce the lithium aluminum hydride, a fire ignited the funnel, [MCA Case History 1182(1966)]. |
|
Hazard |
Moderate fire risk. |
|
Health Hazard |
If ingested causes nausea, vomiting, cramps, weakness, coma. |
|
Fire Hazard |
Behavior in Fire: Containers may explode in fires. |
|
Flammability and Explosibility |
Highlyflammable |
|
Safety Profile |
An experimental teratogen. Other experimental reproductive effects. Readdy forms an explosive peroxide. A very dangerous fire hazard when exposed to heat, flame, or oxidzers. Mixture with lithium tetrahydroaluminate may ignite orexplode if heated. When heated to decomposition it emits acrid smoke and fumes. See also GLYCOL ETHERS. |
|
Purification Methods |
Traces of water and acidic materials have been removed from it by refluxing with Na, K or CaH2, decanting and distilling from Na, K, CaH2 or LiAlH4. The reaction has been speeded up by using vigorous high-speed stirring with molten potassium. For virtually complete elimination of water, 1,2-dimethoxyethane has been dried with Na-K alloy until a characteristic blue colour is formed in the solvent at Dry-ice/cellosolve temperatures: the solvent is kept with the alloy until distilled for use [Ward J Am Chem Soc 83 1296 1961]. Alternatively, glyme, refluxed with benzophenone and Na-K, is dry enough if, on distillation, it gives a blue colour of the ketyl immediately on addition to benzophenone and sodium [Ayscough & Wilson J Chem Soc 5412 1963]. It has also been purified by distillation under N2 from sodium benzophenone ketyl (see above). [Beilstein 1 IV 2376.] |
InChI:InChI=1/C4H10O2/c1-4(5-2)6-3/h4H,1-3H3
-
The new [Li(DME)3+] salt of the previous...
-
Dimethoxyalkanes and dimethyl alkanediyl...
Use of a water-soluble niobium peroxo co...
The trichlorides of Sm, Gd, Tb, Dy, Ho, ...
Carbonylation of methanol to give acetic...
The behaviour of the methoxymethyl radic...
The selective radical dimerization of di...
Microspheres assembled from carbon nanot...
The catalytic performance of composite c...
An AlPO4-supported nickel catalyst exhib...
Two new barium(II) trichloroacetate comp...
Molybdenum carbyne complexes [RCequiv;Mo...
Complexes of ammonium ions RNH3+ (R = CH...
A series of CuCoM (M=Fe, Cr, Ga and Al) ...
We report the formation and full charact...
Catalytic conversion of CO2 into methano...
This disclosure provides processes for f...
2-methoxy-ethanol

1-butyl-1-nitrosourea

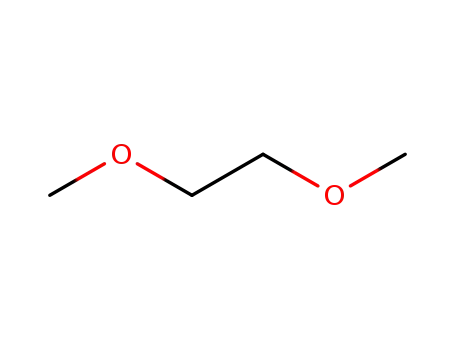
1,2-dimethoxyethane

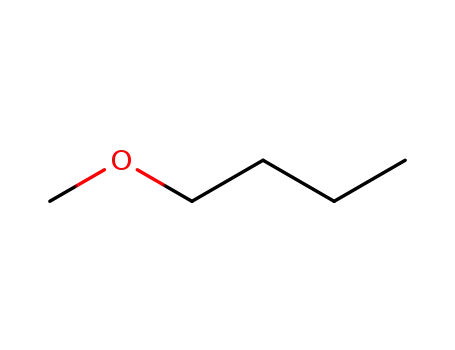
butyl methyl ether

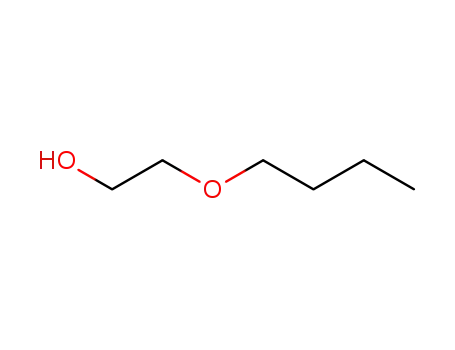
2-Butoxyethanol

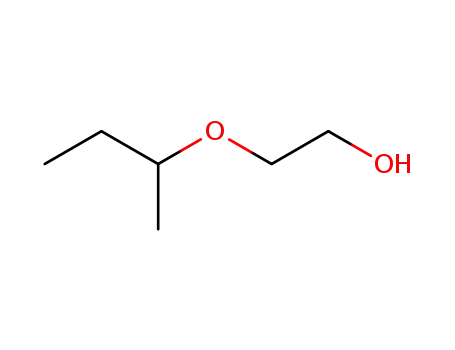
2-(1-Methylpropoxy)ethanol

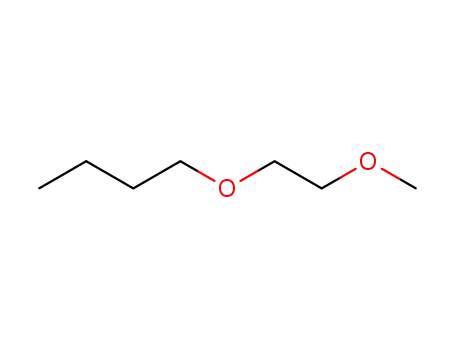
1-(2-methoxy-ethoxy)-butane

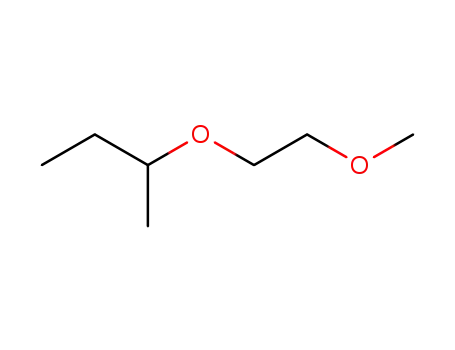
2-(2-Methoxyethoxy)butan
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
for 0.0833333h;
Product distribution;
Mechanism;
Ambient temperature;
further reagent: NaHCO3, further reactions with EtOCH2CH2OH, MeOH-oxirane, -oxetane, -tetrahydrofuran;
|
74.4 % Chromat. 14.2 % Chromat. 0.5 % Chromat. 2.9 % Chromat. 7.2 % Chromat. 0.5 % Chromat. |
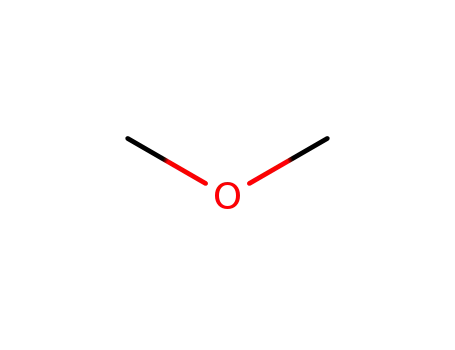
Dimethyl ether

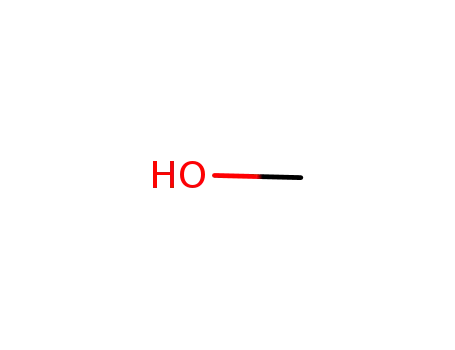
methanol

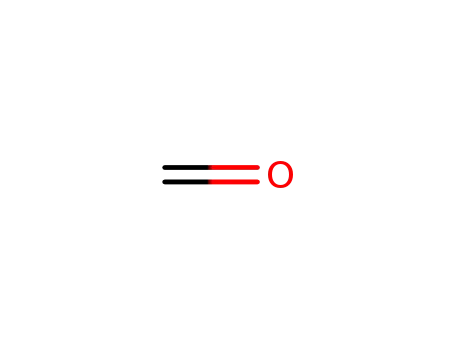
formaldehyd


1,2-dimethoxyethane

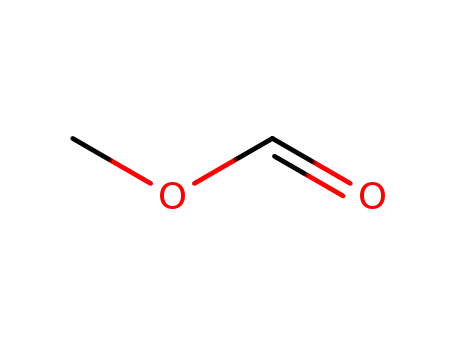
Methyl formate
| Conditions | Yield |
|---|---|
|
With
oxygen;
at 325 ℃;
Reagent/catalyst;
Temperature;
Catalytic behavior;
Flow reactor;
Gas phase;
|

2-methoxy-ethanol
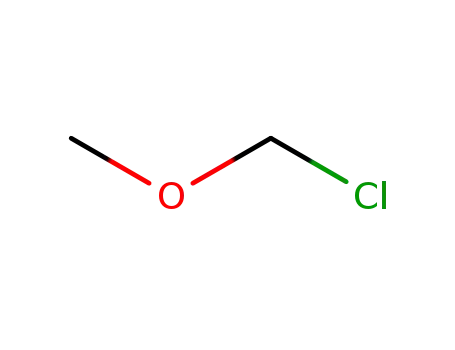
chloromethyl methyl ether
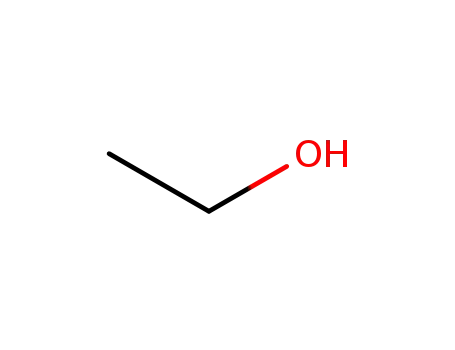
ethanol
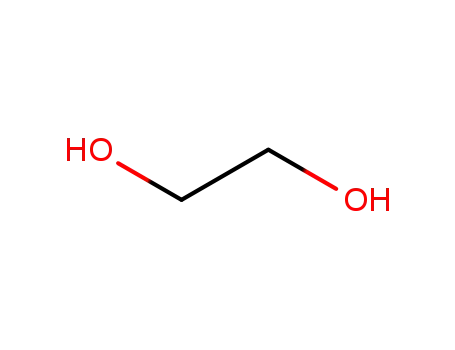
ethylene glycol
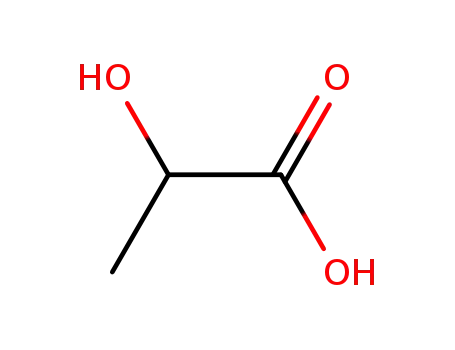
LACTIC ACID
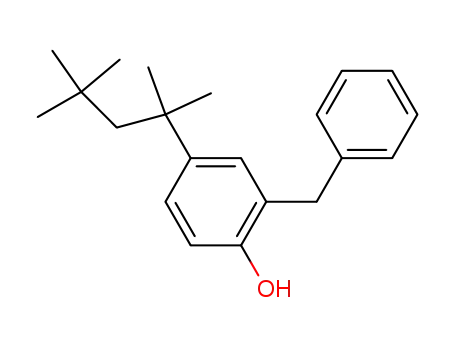
2-Benzyl-4-(1,1,3,3-tetramethyl-butyl)-phenol
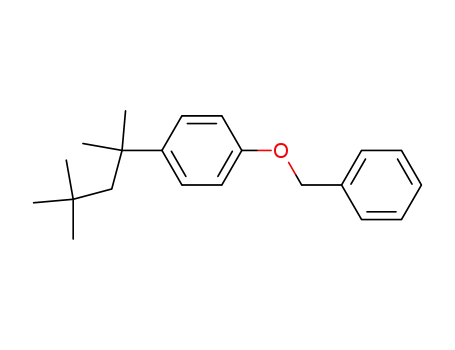
GRI 511254
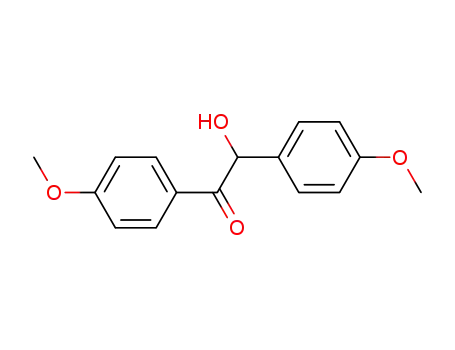
4,4'-dimethoxybenzoin