Your Location:Home > Products > organic chemicals > Ethylene Glycol Monoethyl Ether (EE)
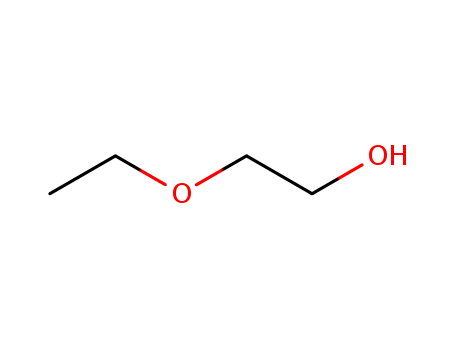


CasNo: 110-80-5
MF: C4H10O2
Appearance: clear, colorless, nearly odorless liquid
|
Chemical Description |
2-ethoxyethanol is a solvent used in organic synthesis. |
|
Air & Water Reactions |
Flammable. Water soluble. |
|
Reactivity Profile |
ETHYLENE GLYCOL MONOMETHYL ETHER may react with oxidizing materials, i.e. hydrogen peroxide, to form peroxides. 2-Ethoxyethanol dissolves many oils, resins and waxes. |
|
Hazard |
Toxic by skin absorption. Moderate fire risk. |
|
Health Hazard |
Some eye irritation. Inhalation of vapors causes irritation of nose. |
|
Fire Hazard |
Special Hazards of Combustion Products: Toxic gases, such as carbon monoxide, may be produced in fire. |
|
Chemical Reactivity |
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. |
|
Safety Profile |
Moderately toxic by ingestion, skin contact, intravenous, and intraperitoneal routes. Mildly toxic by inhalation and subcutaneous routes. An experimental teratogen. Other experimental reproductive effects. A mild eye and skin irritant. Combustible when exposed to heat or flame; can react with oxidzing materials. Moderate explosion hazard in the form of vapor when exposed to heat or flame. Mxture with hydrogen peroxide + polyacrylamide gel + toluene is explosive when dry. To fight fire, use alcohol foam, dry chemical. See also GLYCOL ETHERS. |
|
Potential Exposure |
This material is used as a solvent for nitrocellulose and alkyd resins in lacquers; as a solvent for printing inks; in dyeing leathers and textiles; in the formulation of cleaners and varnish removers; as an anti-icing additive in brake fluids and auto and aviation fuels. |
|
Environmental fate |
Biological. Bridié et al. (1979) reported BOD and COD values of 1.03 and 1.92 g/g using filtered effluent from a biological sanitary waste treatment plant. These values were determined using a standard dilution method at 20 °C for a period of 5 d. When a sewage seed was used in a separate screening test, a BOD value of 1.27 g/g was obtained. Similarly, Heukelekian and Rand (1955) reported a 5-d BOD value of 1.42 g/g which is 72.4% of the ThOD value of 1.96 g/g. Photolytic. Grosjean (1997) reported a rate constant of 1.87 x 10-11 cm3/molecule?sec at 298 K for the reaction of 2-ethoxyethanol and OH radicals in the atmosphere. Based on an atmospheric OH radical concentration of 1.0 x 106 molecule/cm3, the reported half-life of methanol is 0.35 d (Grosjean, 1997). Stemmler et al. (1996) reported a rate constant of 1.66 x 10-11 cm3/molecule?sec for the OH radical-initiated oxidation of 2-ethoxyethanol in synthetic air at 297 K and 750 mmHg. Major reaction products identified by GC/MS (with their yields) were ethyl formate, 34%; ethylene glycol monoformate, 36%; ethylene glycol monoacetate, 7.8%; and ethoxyacetaldehyde, 24%. Chemical/Physical. 2-Ethoxyethanol will not hydrolyze (Kollig, 1993). At an influent concentration of 1,024 mg/L, treatment with GAC resulted in an effluent concentration of 886 mg/L. The adsorbability of the carbon used was 28 mg/g carbon (Guisti et al., 1974). |
|
Shipping |
UN1171 Ethylene glycol monoethyl ether, Hazard Class: 3; Labels: 3-Flammable liquid. |
|
Purification Methods |
Dry it with CaSO4 or K2CO3, filter and fractionally distil it. Peroxides can be removed by refluxing with anhydrous SnCl2 or by filtration under slight pressure through a column of activated alumina. [Beilstein 1 IV 2377.] |
|
Toxicity evaluation |
The toxicity associated with 2-ethoxyethanol is likely caused more by the primary metabolite, ethoxyacetic acid, than by the parent compound. The metabolites have a longer half-life implying a higher accumulation following repeated exposures. Both in vitro and in vivo studies have shown toxic effects from administration of the metabolites that were not seen at higher doses of the parent. Developmental and male reproductive toxicity has been widely documented for several compounds in the glycol ether family, and potency is associated with the length of the hydrocarbon chain: the shorter the chain, the more potent the developmental and reproductive effects. Despite the vast collection of toxicity studies conducted internationally, the exact mechanism of developmental and reproductive toxicity is not well understood. A potential mechanism for the male reproductive toxicity is direct action on Sertoli and/or germ cells by ethoxyacetic acid. The testes have relatively high levels of cytochrome P450 and are an active site of metabolism. Investigators have found that ethoxyacetic acid can cause degeneration of spermatocytes in vitro, and damage to spermatocytes seen in vivo can be suppressed when metabolism of 2-ethoxyethanol is inhibited. |
|
Incompatibilities |
May form explosive mixture with air. Strong oxidizers may cause fire and explosions. Attacks some plastics, rubber and coatings. Able to form peroxides. Incompatible with strong acids; aluminum and its alloys |
|
Waste Disposal |
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal |
|
Physical properties |
Clear, colorless liquid with a sweetish odor. Experimentally determined detection and recognition odor threshold concentrations were 1.1 mg/m3 (300 ppbv) and 2.0 mg/m3 (540 ppbv), respectively (Hellman and Small, 1974). |
|
Definition |
ChEBI: A hydroxyether that is the ethyl ether derivative of ethylene glycol. |
|
General Description |
A clear colorless liquid. Flash point of 120°F. Less dense than water. Its vapors are heavier than air. |
InChI:InChI=1/C4H10O.C2H6O2/c1-3-5-4-2;3-1-2-4/h3-4H2,1-2H3;3-4H,1-2H2
The kinetics of the nitroso group transf...
-
The valorization of lignin has significa...
Etherification of ethylene glycol with m...
The reactions of [WCl6] with a series of...
A versatile heterogeneous photocatalysis...
lignosulfonate

2-ethoxy-ethanol

formic acid

succinic acid

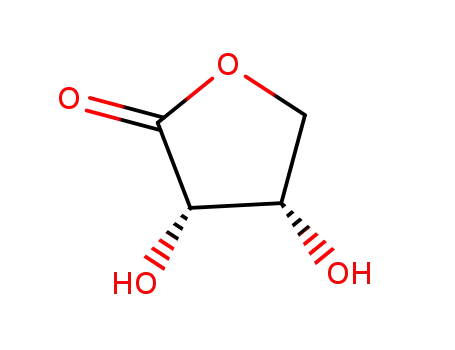
erythrono-1,4-lactone

![7,7-dichloro-3,4-epoxy-4-isopropyl-1-methylbicyclo[4.1.0]heptane](/upload/2025/4/c707b118-754b-434b-997f-64d8bd9a71f3.png)
7,7-dichloro-3,4-epoxy-4-isopropyl-1-methylbicyclo[4.1.0]heptane

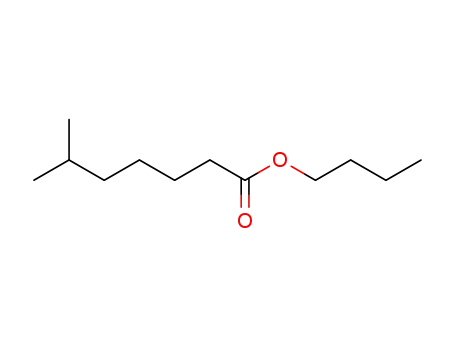
butyl 6-methylheptanoate

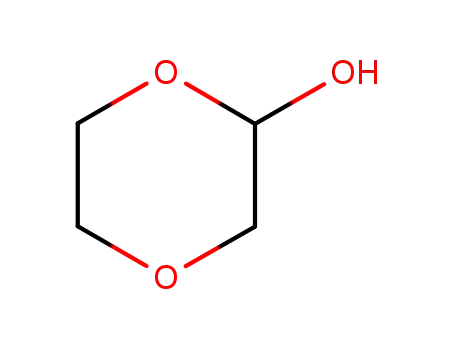
1,4-dioxane-2-ol

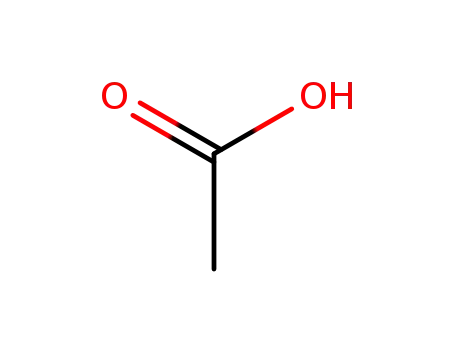
acetic acid

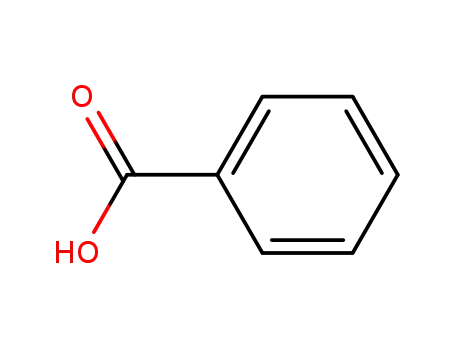
benzoic acid

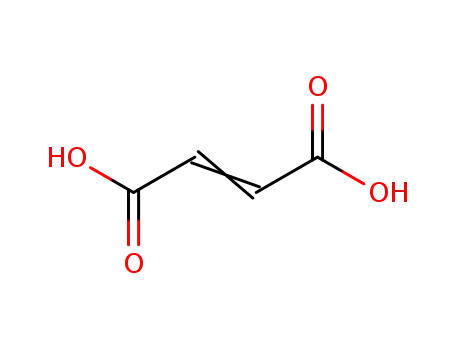
2-butenedioic acid
| Conditions | Yield |
|---|---|
|
With
O4V(3-)*Bi(3+);
In
aq. buffer;
for 24h;
pH=8.2;
Electrochemical reaction;
Irradiation;
|
![[1,3]-dioxolan-2-one](/upload/2025/4/a00019d0-f15f-44d4-a13e-49f58e131ed8.png)
[1,3]-dioxolan-2-one

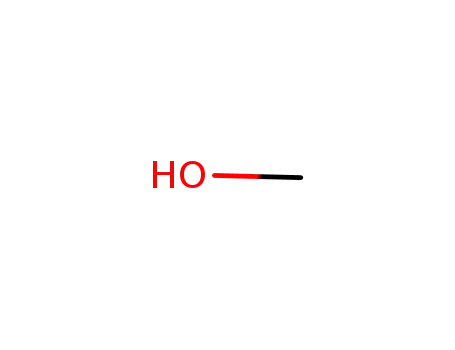
methanol


2-ethoxy-ethanol

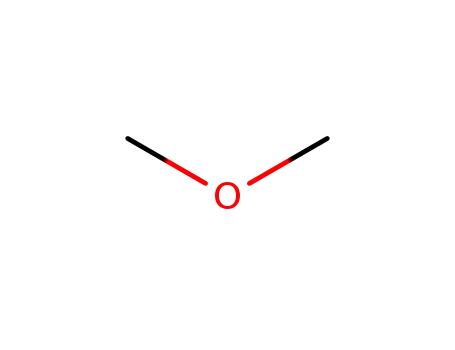
Dimethyl ether

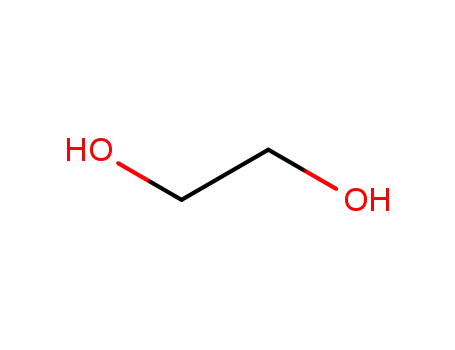
ethylene glycol

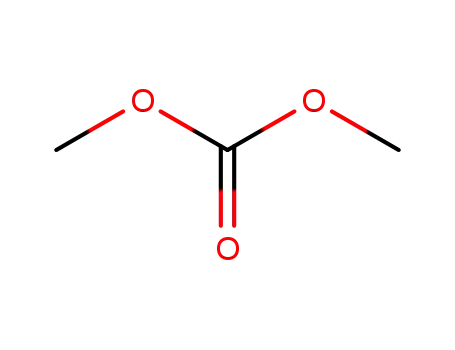
carbonic acid dimethyl ester
| Conditions | Yield |
|---|---|
|
With
carbon dioxide;
at 180 ℃;
for 4h;
under 5175.52 Torr;
Autoclave;
|
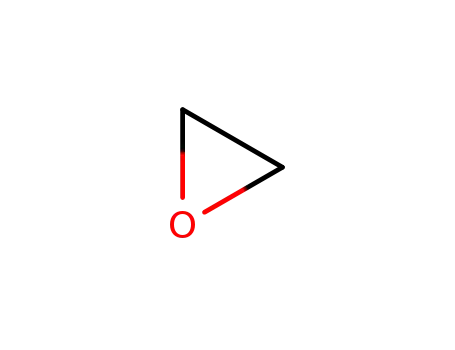
oxirane
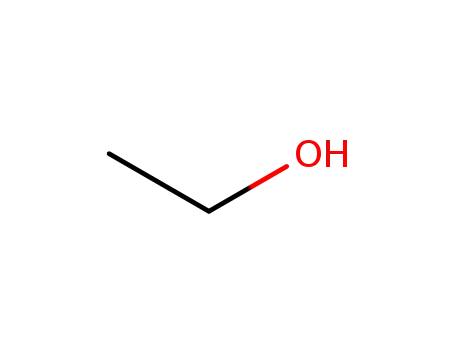
ethanol
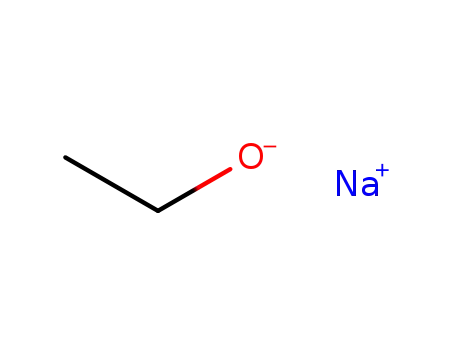
sodium ethanolate
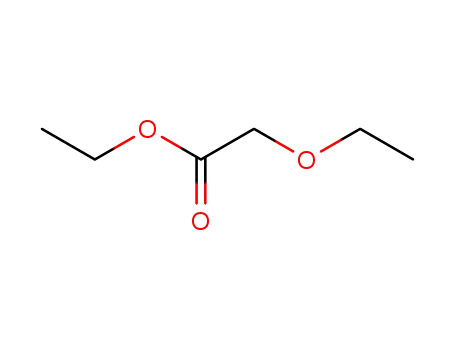
ethyl 2-ethoxyethanoate
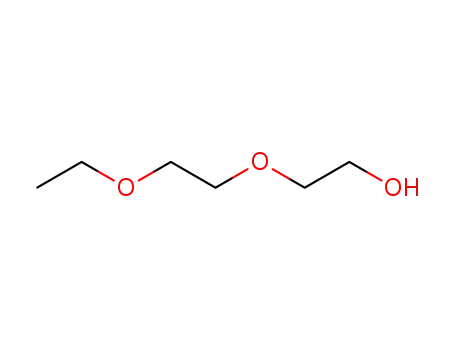
ethoxyethoxyethanol
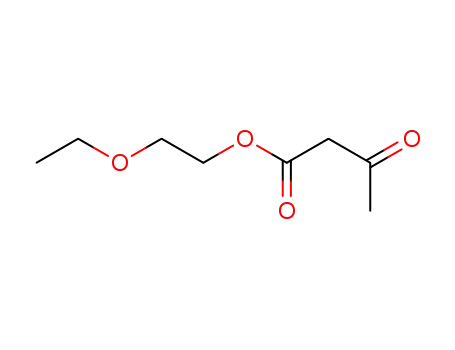
acetoacetic acid-(2-ethoxyethyl)-ester
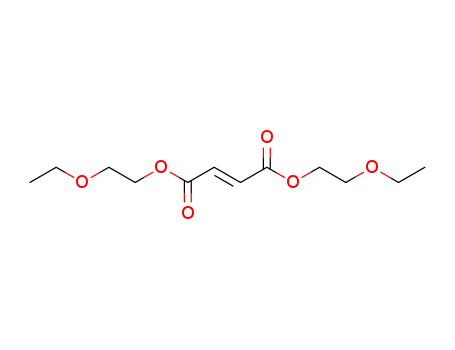
bis-(2-ethoxy-ethyl)-fumarate
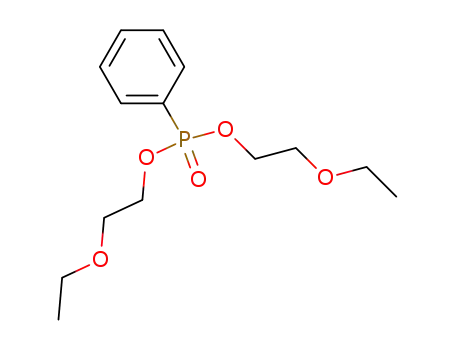
phenyl-phosphonic acid bis-(2-ethoxy-ethyl ester)