Your Location:Home > Products > organic chemicals > 2-PROPOXYETHANOL (EP)
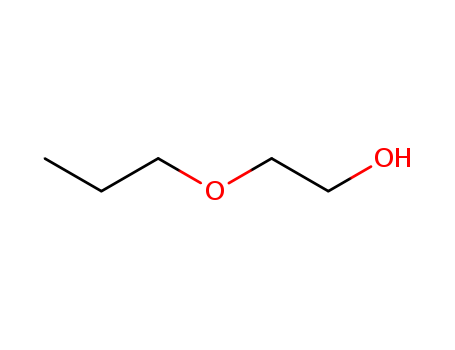


CasNo: 2807-30-9
MF: C5H12O2
Appearance: oily colourless odourless liquid
|
Reactivity Profile |
Ethers, such as 2-PROPOXYETHANOL can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert. |
|
Health Hazard |
VAPOR: Irritating eyes and nose. LIQUID: Can cause corneal damage. INHALATION: Can cause toxic effects. SKIN: Contact can cause toxic effects. |
|
Fire Hazard |
Combustible: Carbon dioxide and carbon monoxide may be produced in a fire. |
|
Safety Profile |
Moderately toxic by ingestion and skin contact. Mildly toxic by inhalation. An experimental teratogen. Experimental reproductive effects. Some glycol ethers have dangerous human reproductive effects. A skin and severe eye irritant. Flammable; can react with oxilzing materials. When heated to decomposition it emits acrid smoke and irritating fumes. See also GLYCOL ETHERS. |
|
General Description |
Liquid; colorless; mild, rancid odor; floats and mixes with water. |
InChI:InChI=1/C6H14O.C2H6O2/c1-3-5-7-6-4-2;3-1-2-4/h3-6H2,1-2H3;3-4H,1-2H2
A liquid phase hydrogenolysis of acetal ...
The phase behavior and microstructure of...
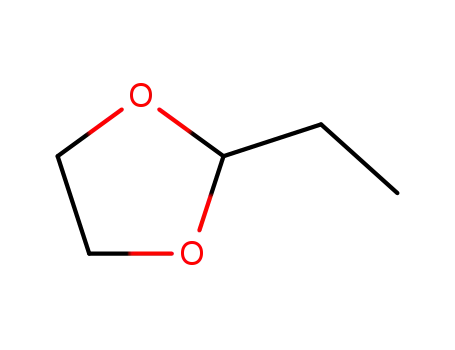
2-ethyl-1,3-dioxolane

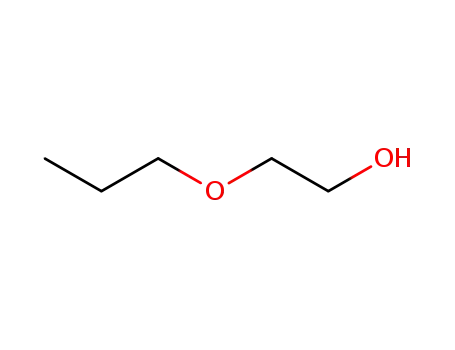
2-propoxyethanol

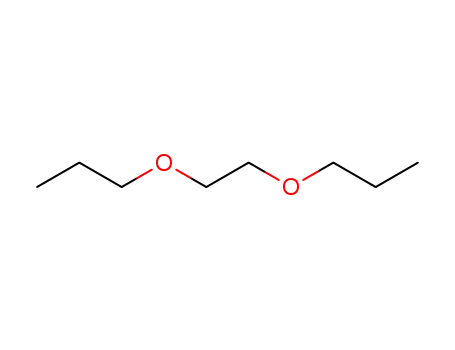
1,2-di-n-propoxyethane
| Conditions | Yield |
|---|---|
|
With
hydrogen;
5%-palladium/activated carbon;
In
ethylene glycol;
at 200 ℃;
for 1h;
under 25802.6 Torr;
Autoclave;
Inert atmosphere;
|
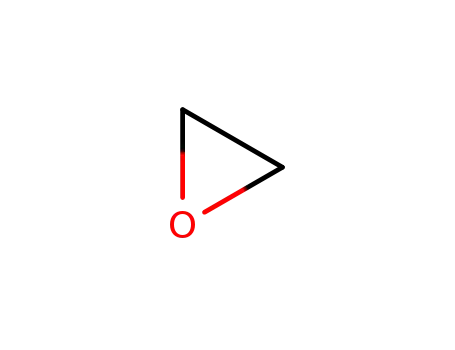
oxirane

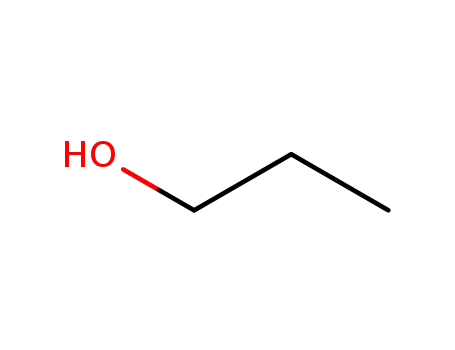
propan-1-ol


2-propoxyethanol
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
|

oxirane

propan-1-ol
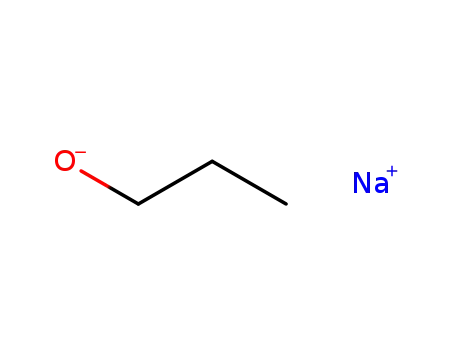
sodium n-propoxide
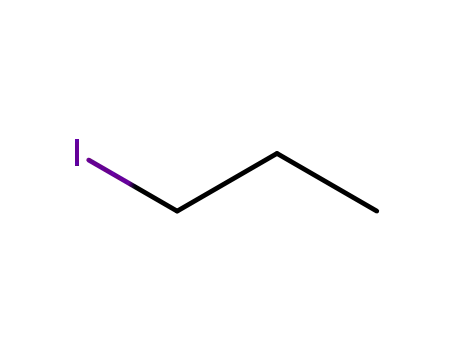
1-iodo-propane
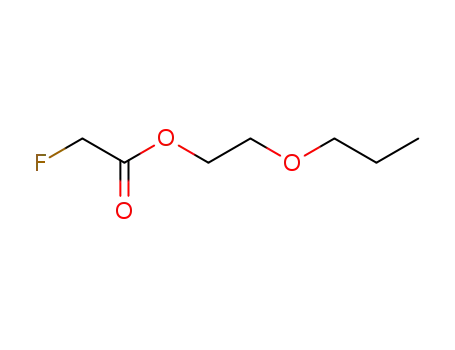
fluoro-acetic acid-(2-propoxy-ethyl ester)
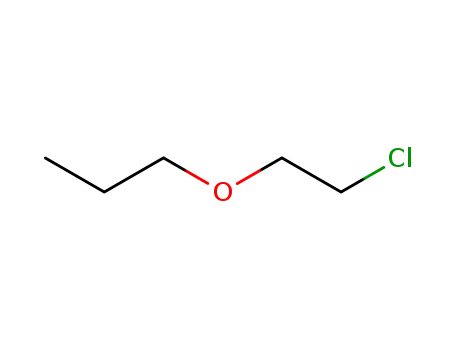
2-chloroethyl propyl ether
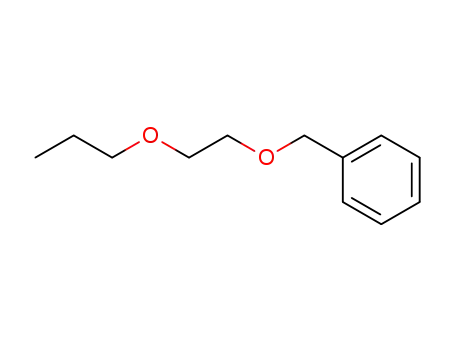
[(2-propoxyethoxy)methyl]benzene
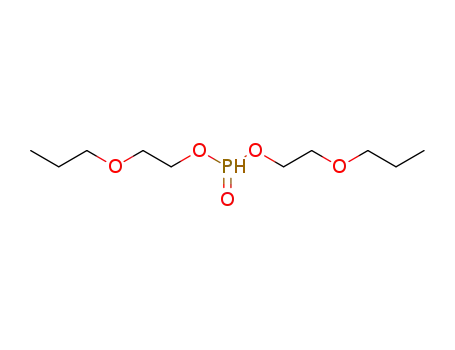
phosphonic acid bis-(2-propoxy-ethyl) ester