Your Location:Home > Products > organic chemicals > TRIETHYLENE GLYCOL MONOMETHYL ETHER (TM)
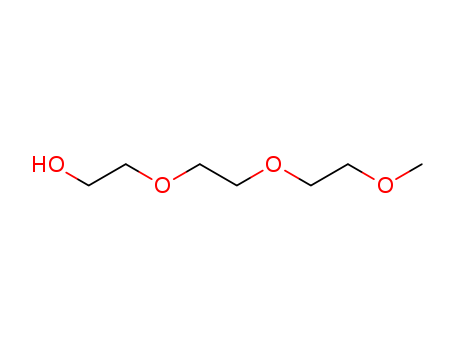


CasNo: 112-35-6
MF: C7H16O4
Appearance: Colorless odorless liquid
|
Air & Water Reactions |
Ethers tend to form unstable peroxides when exposed to oxygen. Ethyl, isobutyl, ethyl tert-butyl, and ethyl tert-pentyl ether are particularly hazardous in this respect. Ether peroxides can sometimes be observed as clear crystals deposited on containers or along the surface of the liquid. |
|
Reactivity Profile |
Ethers, such as TRIETHYLENE GLYCOL MONOMETHYL ETHER, can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert. |
|
Health Hazard |
No appreciable hazard in ordinary handling or use. |
|
Safety Profile |
Mildly toxic by ingestion and skincontact. A skin and eye irritant. Many glycol ethercompounds have dangerous human reproductive effects.Combustible when exposed to heat or flame. To fight fire,use alcohol foam, dry chemical. When heated todecomposit |
|
Definition |
ChEBI: A hydroxypolyether that is the monomethyl ether derivative of triethylene glycol. Metabolite observed in cancer metabolism. |
|
General Description |
Colorless odorless liquid. |
InChI:InChI=1/C7H16O4/c1-9-4-5-11-7-6-10-3-2-8/h8H,2-7H2,1H3
Squaraines are fluorescent, near-IR dyes...
Monoalkyl ethers of ethylene and triethy...
-
An amphiphilic calix[4]arene bearing bra...
Novel water soluble robust fluorescent c...
The inclusion complexes of a new family ...
The synthesis of perfectly planar, bis-s...
3-Hydroxybenzyl alcohol

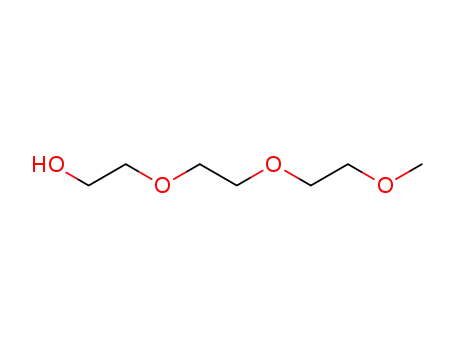
triethylene glucol monomethyl ether

3-Phenoxybenzyl alcohol
| Conditions | Yield |
|---|---|
|
|
87% |
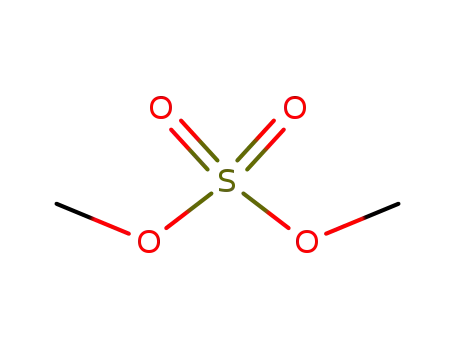
dimethyl sulfate

![2,2'-[1,2-ethanediylbis(oxy)]bisethanol](/upload/2025/4/bcf69c89-7cd4-4682-9fe0-13705a688959.png)
2,2'-[1,2-ethanediylbis(oxy)]bisethanol


triethylene glucol monomethyl ether
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
at 120 ℃;
for 4h;
|
59% |
|
With
sodium hydroxide;
at 120 ℃;
|
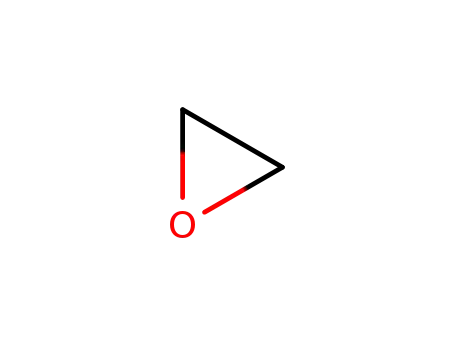
oxirane
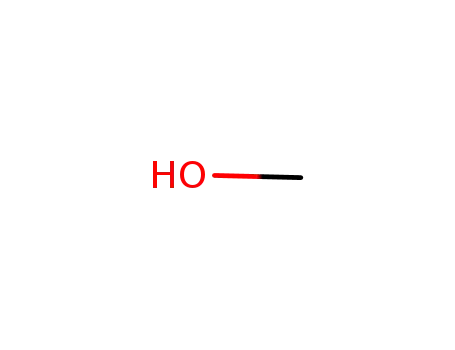
methanol
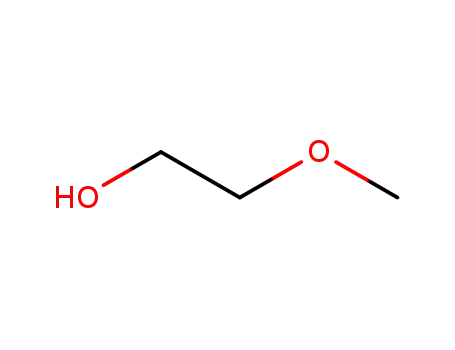
2-methoxy-ethanol
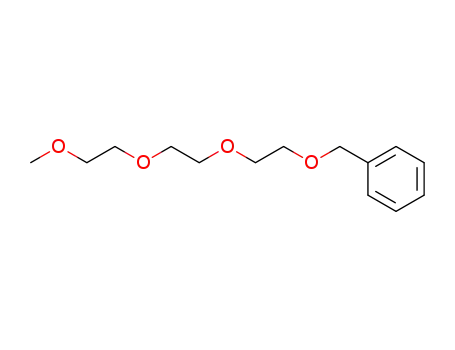
triethylene glycol benzyl methyl ether
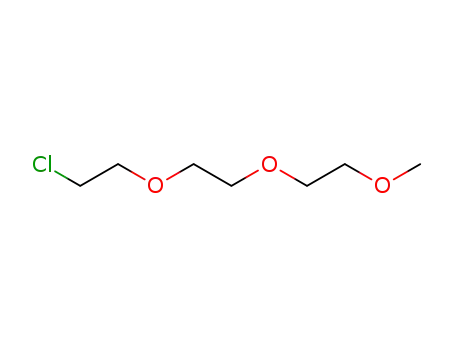
1-chloro-3,6,9-trioxadecane
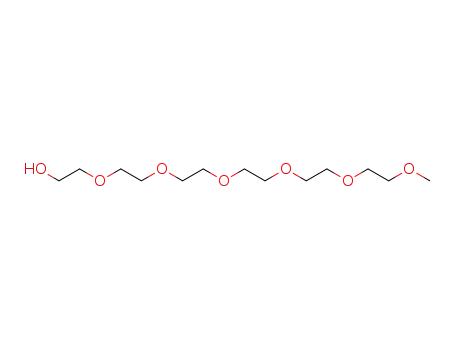
hexaethylene glycol monomethyl ether
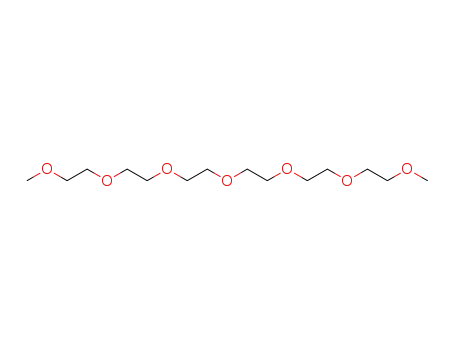
2,5,8,11,14,17,20-heptaoxahenicosane
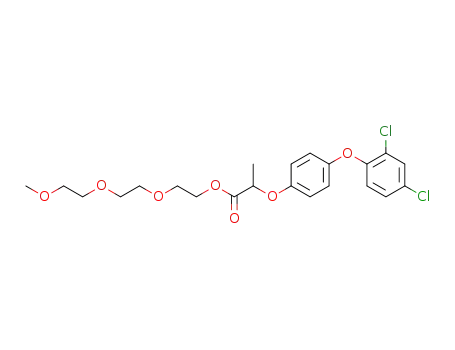
2-[4-(2,4-Dichloro-phenoxy)-phenoxy]-propionic acid 2-[2-(2-methoxy-ethoxy)-ethoxy]-ethyl ester