Your Location:Home > Products > organic chemicals > 1-Methoxy-2-propanol (PM)
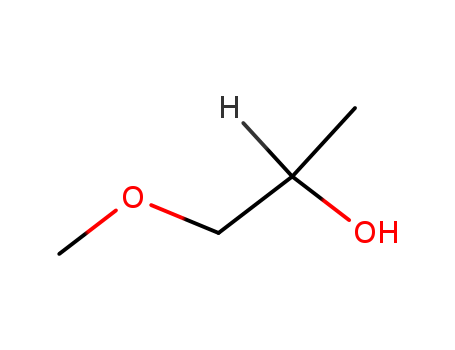


CasNo: 107-98-2
MF: C4H10O2
Appearance: colourless liquid
|
General Description |
A colorless liquid. Flash point near 89°F. Less dense than water. Contact irritates skin, eyes and mucous membranes. Prolonged exposure to vapors may cause coughing, shortness of breath, dizziness and intoxication. Vapors heavier than air. Used as a solvent and as an antifreeze agent. |
|
Air & Water Reactions |
Highly flammable. Soluble in water. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979 p.151-154, 164]. |
|
Reactivity Profile |
1-Methoxy-2-propanol is a methoxy alcohol derivative. The ether being relatively unreactive. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. |
|
Hazard |
Flammable, moderate fire risk. TLV: 100 ppm; STEL 150 ppm. |
|
Health Hazard |
VAPOR: Irritating to eyes, nose, and throat. LIQUID: Irritating to skin and eyes. |
|
Fire Hazard |
FLAMMABLE. Flashback along vapor trail may occur. Vapor may explode if ignited in an enclosed area. |
|
Flammability and Explosibility |
Flammable |
|
Safety Profile |
Moderately toxic by intravenous route. Mildly toxic by ingestion, inhalation, and skin contact. Human systemic effects by inhalation: general anesthesia, nausea. A skin and eye irritant. An experimental teratogen. Many glycol ethers have dangerous human reproductive effects. Very dangerous fire hazard when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. Used as a solvent and in solvent-sealing of cellophane. See also GLYCOL ETHERS and ETHYLENE GLYCOL MONOMETHYL ETHER. |
|
Synthesis |
1-Methoxy-2-propanol is used as a reagent in the synthesis of 2-amino-3-carboxy-4-phenylthiophenes, which acts as a protein kinase C inhibitors. It is also used as a reagent in the synthesis of metolachlor. |
|
Potential Exposure |
Propylene glycol monomethyl ether is used as a solvent for coatings; cellulose esters and acrylics; acrylics dyes; inks, and stains. It may also be used as a heat-transfer fluid. |
|
Shipping |
UN3092 1-Methoxy-2-propanol, Hazard Class: 3; Labels: 3-Flammable liquid. |
|
Toxicity evaluation |
Contact irritates skin, eyes and mucous membranes. Prolonged exposure to vapors may cause coughing, shortness of breath, dizziness and intoxication. Vapors heavier than air. Used as a solvent and as an antifreeze agent. The oral LD50 of rats was 6.6g/kg. The skin irritation is not obvious, but the toxic dose can be absorbed through the skin. The main manifestations of animal poisoning were inhibition and incomplete anesthesia. Half of the rats died when they were exposed to steam concentration of 40.18g/m3 for 5 ~ 6 hours. the impact of 1-methoxypropanol-2 (MEP) for the stimulation of an inflammatory response in human respiratory mucosa, we exposed 22 primary cell cultures of nasal respiratory epithelia of healthy individuals to MEP concentrations at the level of the German MAK-value (100ppm) and to the 10-fold concentration (1000ppm). |
|
Incompatibilities |
Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid chlorides, acid anhydrides, isocya- nates, aluminum, and copper. Hygroscopic (i.e., absorbs moisture from the air). May slowly form reactive peroxides during prolonged storage or on exposure to air and light. |
|
Waste Disposal |
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. |
InChI:InChI:1S/C4H10O2/c1-4(5)3-6-2/h4-5H,3H2,1-2H3
-
Crown ethers (i.e. [2,4]-dibenzo-18-crow...
A series of basic and acidic ionic liqui...
β-Hydroxy esters are considered as poten...
Different bi-functional materials (Pd(Au...
Ionic liquid 1,1,3,3-tetramethylguanidiu...
The properties of TS-1 catalyst in conti...
Defects in metal-organic frameworks (MOF...
To mitigate environmental concern and en...
To ensure the quasi-irreversibility of t...
Bimetallic Al-La oxides were conjointly ...
The fluorine-modified Mg-Al mixed oxides...
The epoxidation of propylene with hydrog...
A novel reagent, Bu3SnH/Bu3SnI-phosphine...
A facile method for the size-controllabl...
This work describes the selective H2O2 d...
Tetramethylguanidine-based ionic liquids...
In this work, we found that MCM-41 prepa...
Protonation of chiral porous materials i...
A simple and effective approach for the ...
-
-
ZnO/MMT nanocomposite as sonocatalyst wa...
Information about the degree of substitu...
Al2O3/MgO materials with various Mg/Al m...
The fact that the homogeneous acid catal...
The aim of this study was to investigate...
The basicity of methylammonium-faujasite...
-
The direct synthesis of dimethyl carbona...
In this work, we immobilized ionic liqui...
The kinetics of propylene oxidation into...
Nano metal oxides such as Fe2O3, Fe3O4, ...
The synthesis of cyclic carbonates or di...
Mg-Al hydrotalcites with different Mg/Al...
Alcoholysis of propylene oxide (PO) is a...
Glycols and ethoxy– and propoxy–alcohols...
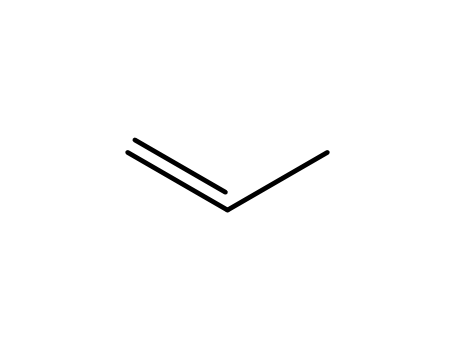
propene

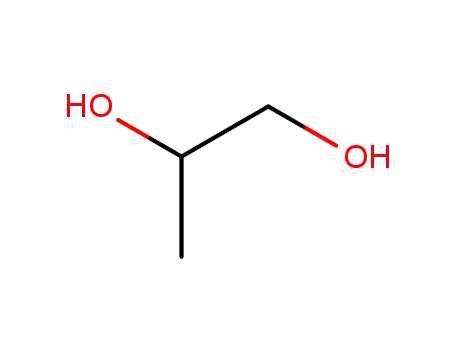
propylene glycol

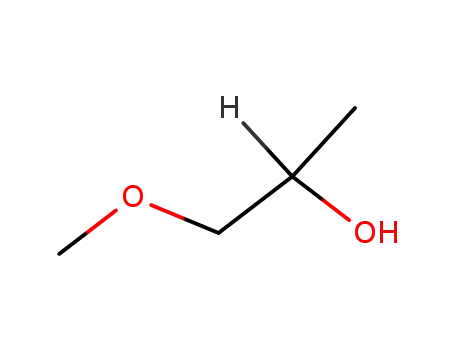
1-methoxy-2-propanol

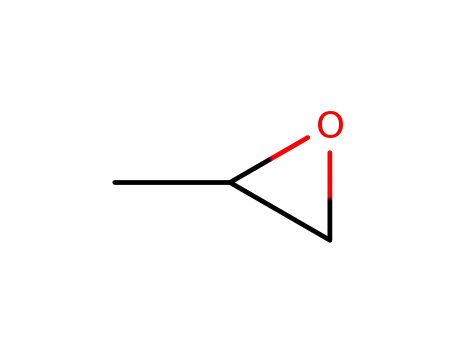
methyloxirane
| Conditions | Yield |
|---|---|
|
With
pyridine N-oxide; dihydrogen peroxide;
methyltrioxorhenium(VII);
In
methanol; water;
at 40 ℃;
for 3h;
under 36201.3 Torr;
|
72.73% 1.85% 0.59% |
|
With
dihydrogen peroxide;
In
methanol;
at 49.84 ℃;
under 5250.53 Torr;
Green chemistry;
|

propene


propylene glycol


1-methoxy-2-propanol

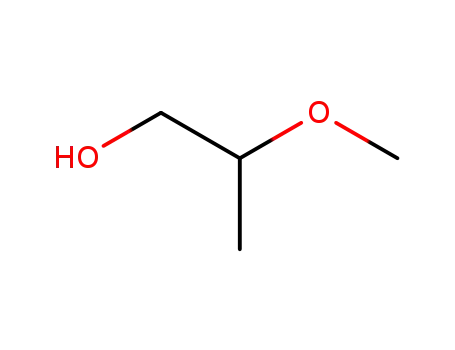
2-methoxypropanol


methyloxirane
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide;
titanium-silicate;
In
methanol;
at 40 ℃;
|
92% |
|
With
dihydrogen peroxide;
titanium-silicate;
In
methanol;
at 40 ℃;
|
78% |
|
With
dihydrogen peroxide;
titanium-silicate;
In
water;
at 30 - 80 ℃;
under 18751.9 Torr;
pH=4.5;
|
61% |
|
With
dihydrogen peroxide;
titanium silicate;
In
methanol; tert-butyl methyl ether; water;
at 60 ℃;
for 4h;
under 3750.38 Torr;
pH=4.6;
|
|
|
With
dihydrogen peroxide;
titanium silicate;
In
methanol; tert-butyl methyl ether; water;
at 50 ℃;
for 8h;
under 11251.1 Torr;
pH=4.8;
|
|
|
propene;
With
ammonia; dihydrogen peroxide;
In
methanol; tert-butyl methyl ether; water;
at 45 ℃;
under 2250.23 Torr;
titanium silicate;
at 39 ℃;
for 8h;
pH=5.5 - 8.9;
|
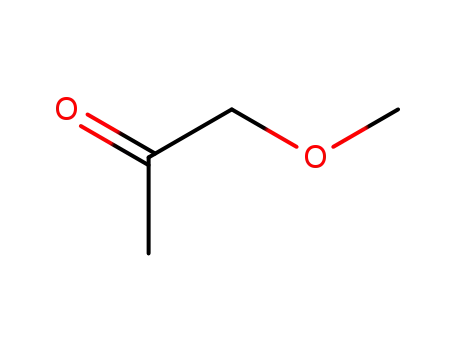
Methoxyacetone
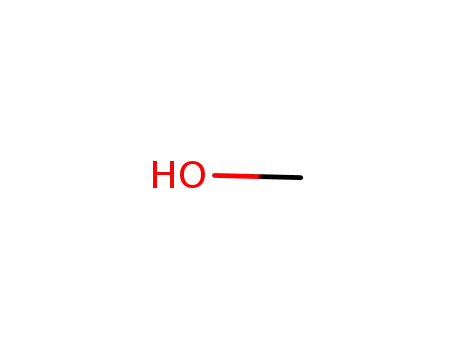
methanol

methyloxirane
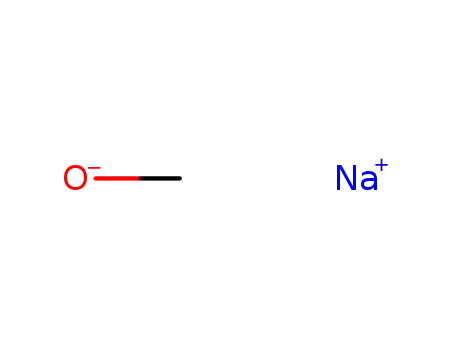
sodium methylate

Methoxyacetone
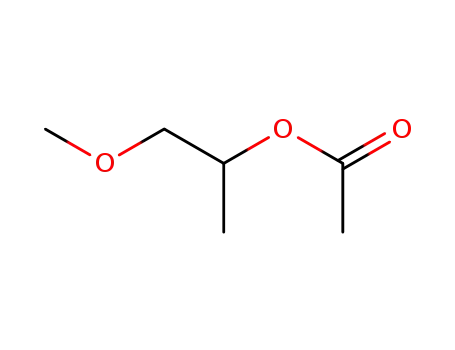
propylene glycol methyl ether acetate
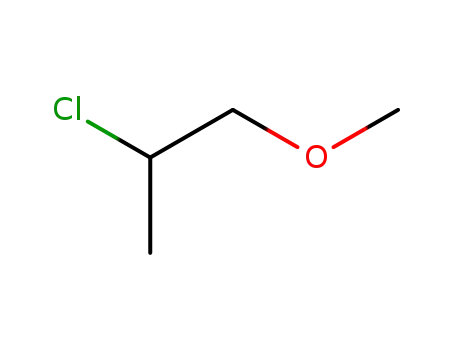
2-Chlor-1-propyl-methylether
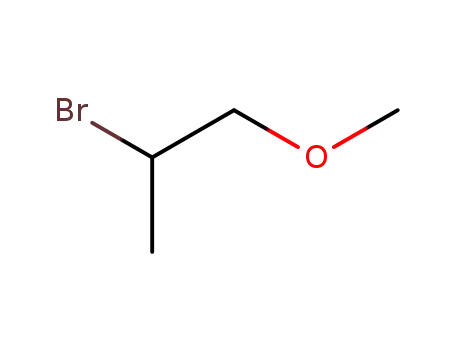
2-Bromo-1-methoxypropane